Tilray: A Global Leader
Tilray is a global leader in cannabis research, cultivation, processing and distribution. We aspire to lead, legitimize and define the future of our industry by building the world’s most trusted cannabis and hemp company.
A proud pioneer, we are the first GMP-certified medical cannabis producer to supply cannabis flower and extract products to tens of thousands of patients, physicians, pharmacies, hospitals, governments and researchers on five continents.
EXPERIENCE
Tilray's experience is unmatched worldwide. Our team of professionals on the ground in 7 countries serves tens of thousands of patients and consumers around the globe. Our GMP-certified cultivation and production facilities are among the most advanced in the world.
PRECISION
Tilray is the first GMP-certified medical cannabis producer to supply cannabis flower and extracts. All of our products are produced with meticulous care to ensure the highest quality, consistency and purity for our patients around the world.
RESEARCH
We are committed to scientific research that leads to an improved quality of life for patients in a time frame that matters. We partner with leading hospitals and universities to advance the clinical applications of cannabinoids.
CARE
Tilray takes tremendous pride in our customer service, patient outreach, and physician interaction. We recognize the importance of tracking potential adverse events as well as therapeutic benefits in order to ensure the safety of our patients.
Precisely formulated Products
Tilray cultivates a wide range of strains to meet patient needs including indicas, sativas, hybrids, and CBD-rich varieties. Medical cannabis is available in two primary forms: extracts and dried flower. Each can be administered or consumed via a variety of methods.
We take a scientific approach to product development. Our product line focuses on active ingredients and standardized, well-defined preparations. We use formulations and delivery formats that are intended to allow for consistent and measured dosing, and we test all of our products for potency and purity. Each of our commercial products are developed with comprehensive analysis and thorough documentation, including stability profiles, certificates of analysis, monographs and drug master files.
Advancing Cannabinoid Science
For the therapeutic value and risks of cannabinoid-based medicines to be fully understood, Tilray believes it is critical to evolve current scientific understanding of the field. Our clinical research program is designed with that in mind.
Participation in clinical trials is a differentiating element of our research and development program. We have developed techniques that achieve clinical grade isolates in order to facilitate participation in clinical trials conducted by select research partners at world-leading hospitals and universities.
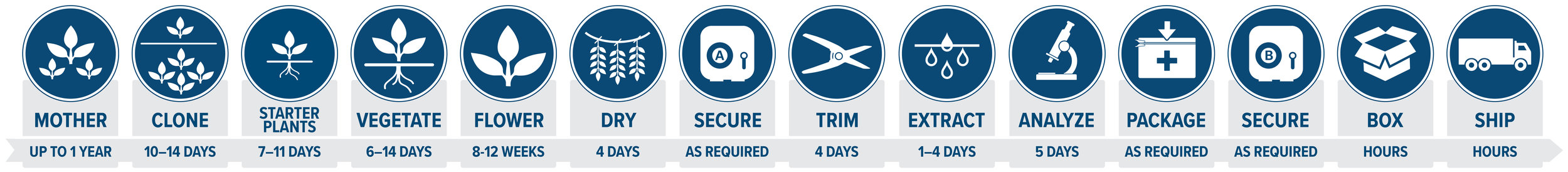
Production Process
Tilray choreographs a complex series of cutting-edge processes to deliver unparalleled product to its patients. From cultivation to shipping, Tilray's team brings precision, professionalism and care to each stage of the process.
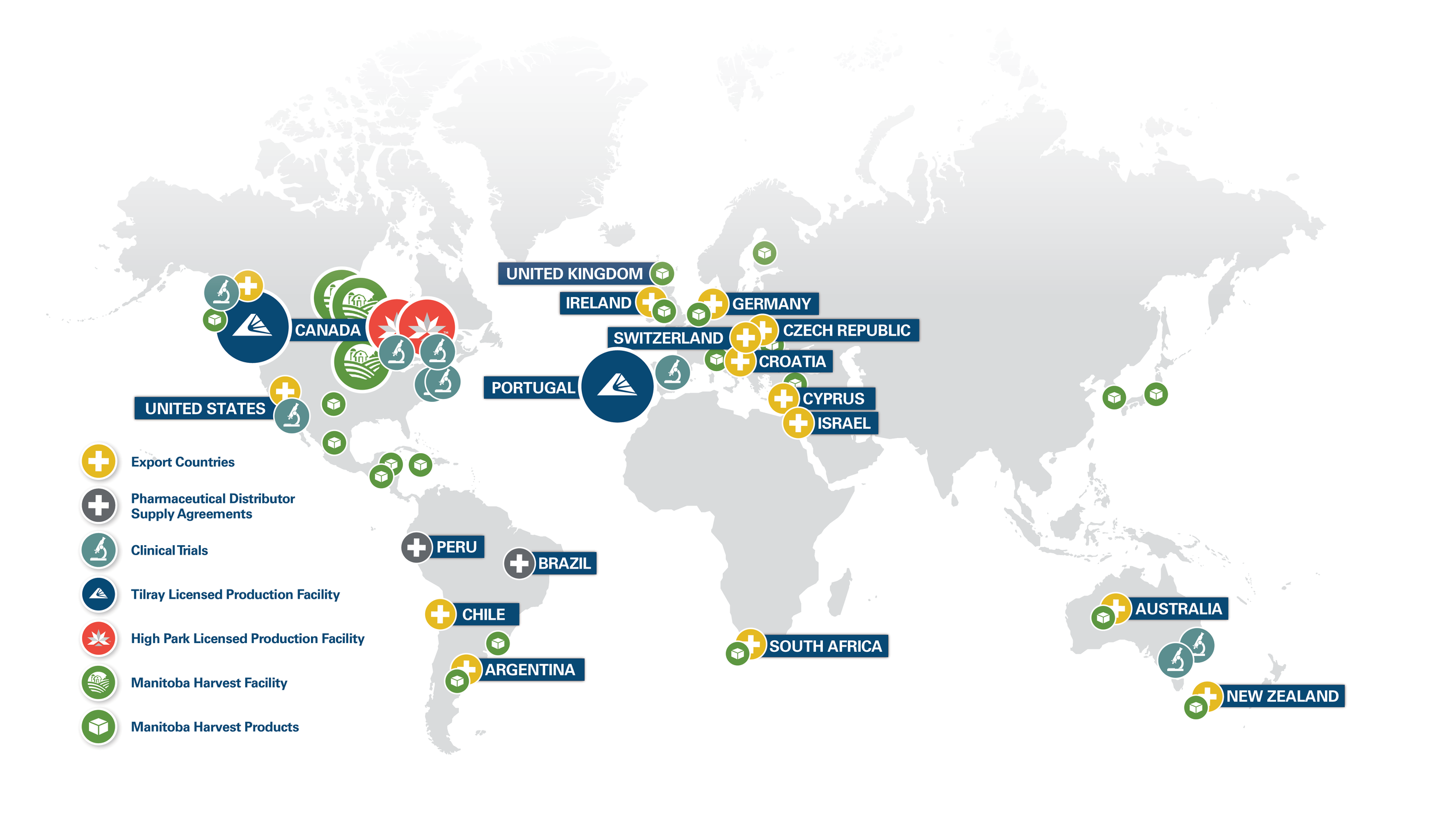
Global Reach
As laws and regulations regarding cannabis and hemp evolve in different jurisdictions, we are actively seeking to expand our operations around the world.
AUSTRALIA & NEW ZEALAND
Tilray was the first company to legally export medical cannabis from North America to Australia and New Zealand. Today, Tilray is one of the leading providers of medical cannabis in Australia and New Zealand for commercial, compassionate access and research purposes.
CANADA
Tilray was one of the first companies to be licensed to produce medical cannabis in Canada. Today, Tilray is the country's leading scientifically rigorous provider of medical cannabis, supplying tens of thousands of patients in every province with consistent access to whole flower, milled blends and oils.
EUROPE
Tilray was the first company to legally export medical cannabis products from North America to Europe. Tilray also has a cultivation license from the Government of Portugal to produce products for the EU market. Tilray products are currently available at pharmacies in countries throughout the EU.
LATIN
AMERICA
Tilray Latin America imports, produces and distributes Tilray branded medical cannabis products in Chile. Tilray Latin America will act as a hub to distribute Tilray products throughout Latin America, strengthening the company’s presence in the region.
Board of Directors
Irwin D. Simon, Chairman of the Board of Directors
An executive with over 30 years of experience building industry-leading, disruptive consumer packaged goods companies from organic and natural foods, dietary supplements, personal care, and cannabis. Before Tilray, Mr. Simon transformed Aphria Inc. into a profitable global cannabis company with leading market share brands. Mr. Simon founded The Hain Celestial Group, Inc. (NASDAQ: HAIN), a leading organic and natural products company, in 1993. As Founder, President, Chief Executive Officer, and Chairman, he led Hain Celestial for more than 25 years and grew the business to $3 billion in net sales with operations in North America, Europe, Asia, and the Middle East, providing consumers with A Healthier Way of Life™. He is also the Executive Chairman of Whole Earth Brands, Inc. (NASDAQ: FREE), a global industry-leading platform focused on the “better for you” consumer packaged goods and ingredients space, and Presiding Director at MDC Partners Inc., a provider of marketing, activation and communications solutions and services.
Mr. Simon serves on the board of directors at Tulane University and the Board of Trustees at Poly Prep Country Day School. A true entrepreneur, Mr. Simon is also the majority owner of the Cape Breton Screaming Eagles, a Quebec Major Junior Hockey League team, and co-owner of St. John’s Edge of the National Basketball League of Canada.
Renah Persofsky , ICD.D, Vice-Chair (Lead Director) and Chair of the Nominating and Governance Committee, Independent Director
Renah Persofsky has over 40 years of business experience. She presently serves as the Board Chair for BookJane, an innovative technology platform that enhances the opportunity of the gig economy in the healthcare space, and as the executive Chair of Green Gruff, a dog wellness company that produces organic and sustainable dog supplements. Renah also serves on the board of Hydrofarm Holdings Group, Inc. America’s oldest and largest independent wholesaler and manufacturer of hydroponics equipment and grow lights. Recently, Renah was appointed to the board of Alkemy, the world’s first plastic mining company. She has been an executive consultant to many iconic brands including Tim Hortons, Canadian Tire, CIBC, Canada Post and Interac, and was an executive officer of the Bank of Montreal. Ms. Persofsky is a global leader in e-commerce and has co-chaired the Canadian Minister’s Advisory Committee on Electronic Commerce, as well as served as a special advisor to the Minister of Foreign Affairs and Trade.
Jodi Butts, Nominating & Governance Committee Member, Independent Director
Jodi Butts is a senior governance consultant at WATSON Advisors Inc., who brings more than 20 years’ experience in governance and law, working with public and private corporations, public sector entitles, member-based organizations, regulatory bodies, and not-for-profit organizations. Jodi brings deep governance expertise gained from her experience as a lawyer, CEO, senior executive, and as a director of public, private, and not-for-profit corporations. Jodi connects governance with people, strategy, and operations to bring a wealth of practical know-how to boards and executive teams. Jodi currently serves as an independent director of Canada Goose Inc. and as chair of The Walrus Board of Directors. She also holds advisory roles with Bayshore Home Healthcare, and the Canadian Centre for the Purpose of the Corporation. Jodi is a retired member of the University of Windsor Board of Governors. Previously, Jodi served as CEO of Rise Asset Development, and Senior Vice-President Corporate Affairs and Operations for Mount Sinai Hospital. Jodi holds a BA (English Literature and History) from the University of Windsor and a Bachelor of Laws from the University of Toronto where she also received her master’s degree in Canadian history.
David Clanachan, Audit Committee Member, Independent Director
David F. Clanachan is Commissioner of the Canadian Premier League, a post he has held since 2018. Mr. Clanachan was also the Chairman of Restaurant Brands International, Canada until 2018. He was named President and Chief Operating Officer of Tim Hortons in 2014, and had more than 35 years with the brand. Mr. Clanachan holds a Bachelor of Commerce degree from the University of Windsor. Mr. Clanachan brings to the Board significant experience in consumer products and services, as well as financial, international growth, and general management experience.
John M. Herhalt Chair of the Audit Committee, Independent Director
John M. Herhalt is a FCPA (FCA) and a retired partner from KPMG and has over 40 years of experience. He has worked across several industry sectors including automotive manufacturing, consumer products, infrastructure, power and utilities, and the public sector. During his time with KPMG, Mr. Herhalt served as Canada’s national advisory leader, national public sector leader, and KPMG International’s global head of infrastructure, government, and health care sectors providing subject matter advice and support to various KPMG member firms and their clients on a variety of projects in the Americas, Europe, Middle East, and Asia. After retiring from KPMG, Mr. Herhalt has continued to provide management consulting services on a part-time basis and serves as a director on several boards.
David Hopkinson, Nominating and Governance Committee & Compensation Committee Member, Independent Director
An accomplished executive with more than 25 years of diverse sports industry experience, Mr. Hopkinson is Executive Vice President of Madison Square Garden Sports and President of Team Business Operations for MSG’s portfolio of teams which include the New York Knicks (NBA), New York Rangers (NHL) and esports businesses Counter Logic Gaming and Knicks Gaming. Prior to joining MSG Sports, David served as Global Head of Partnerships for Real Madrid Club de Futbol in Madrid, Spain from 2018 to 2020. Mr. Hopkinson spent over 20 years with Maple Leaf Sports and Entertainment (MLSE) in Toronto, Canada and in his last role with MLSE, he served as Chief Commercial Officer, responsible for all revenue generation across MLSE’s teams; the Toronto Maple Leafs (NHL), Toronto Raptors (NBA) and Toronto FC (MLS). David has served on the Chancellor’s Advisory Committee at McGill University in Montreal, Canada as well as the Board of Directors of Canada Basketball and Board of Directors of Canada’s Walk of Fame. In 2013, he was awarded the Queen Elizabeth II Diamond Jubilee Medal in recognition of his Service to Canada.
Tom Looney, Audit Committee & Compensation Committee Member, Independent Director
Tom Looney is the former President of Diageo US Spirits & Canada. In this position Mr. Looney maintained full responsibility for the growth and development of the company’s spirits business in the United States & Canada including brands such as Smirnoff, Crown Royal, Baileys, Johnnie Walker, Captain Morgan, and Ketel One. Mr. Looney was also a member of Diageo’s North American Executive Team. Previously, Mr. Looney held the position of President, Diageo Beer Company overseeing US sales, finance, marketing, and innovation teams.